Blog
Specright Continues to Expand and Enhance Features in Product Release 23.0
We are excited to announce new features and enhanced capabilities to the Specright Specification Data Management Platform with Release 23.0. These include Plan for Every Part features, FDA Supplier Recalls/Inspection Data Integrations, UoM Conversion Enhancements, and more. Specright’s latest release is sure to allow customers to keep operating efficiently and driving strategic business goals.
Posted on

The Specright Product Team has been hard at work to release our third major release of 2023, Version 23.0. This release includes new features, improvements to existing features, and addresses issues that customers brought to our attention.. We are committed to providing the best possible solution for our customers, and many of these new and enhanced features are a result of direct customer feedback.
In this blog, we’ll be highlighting the major features we released, but more details about the entirety of our 23.0 release will be addressed in the Product 23 Release webinar.
New features to our Specification Data Management Platform
Revision Management – Change Requests and Mass Update
The Change Requests feature enables users to initiate change requests for a range of actions. In this initial release, we are introducing the ability for users to create change requests specifically for mass updating records. This comprehensive solution includes a streamlined approval process that ensures only authorized changes are made, enhancing control and data integrity in record modifications.
This feature offers a multitude of benefits to our users. First and foremost, it simplifies the process of updating multiple records at once, significantly reducing the time and effort required for data management tasks. The integrated approval process adds an essential layer of accountability and control, preventing unauthorized changes and preserving data accuracy.
Furthermore, it facilitates easy tracking and auditing of changes, supporting compliance and governance requirements.
Ultimately, this feature empowers users to work more efficiently, while maintaining the highest data quality standards, and offers an enhanced user experience within our platform.
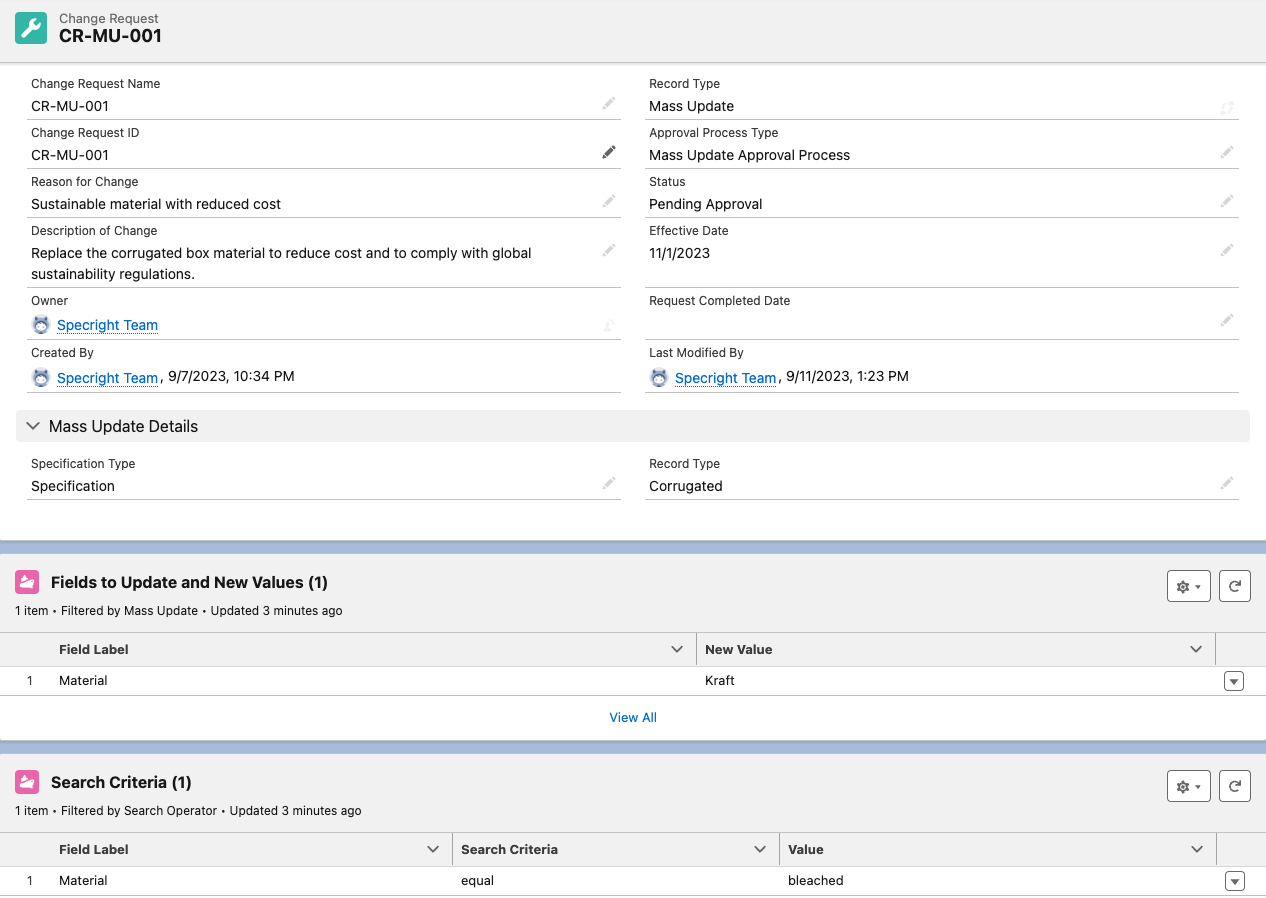
Fig 1: Change Request Details
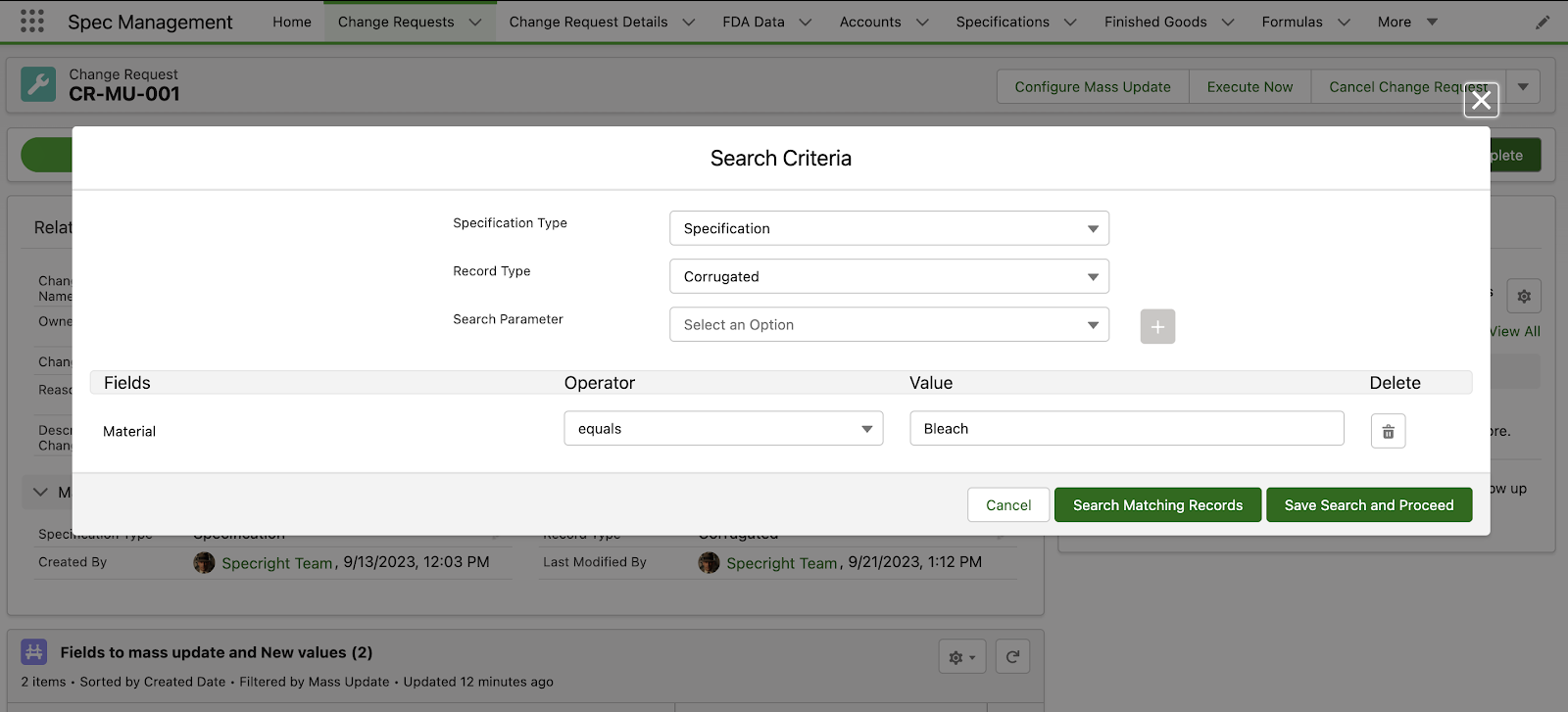
Fig 2: Change Request Search Criteria
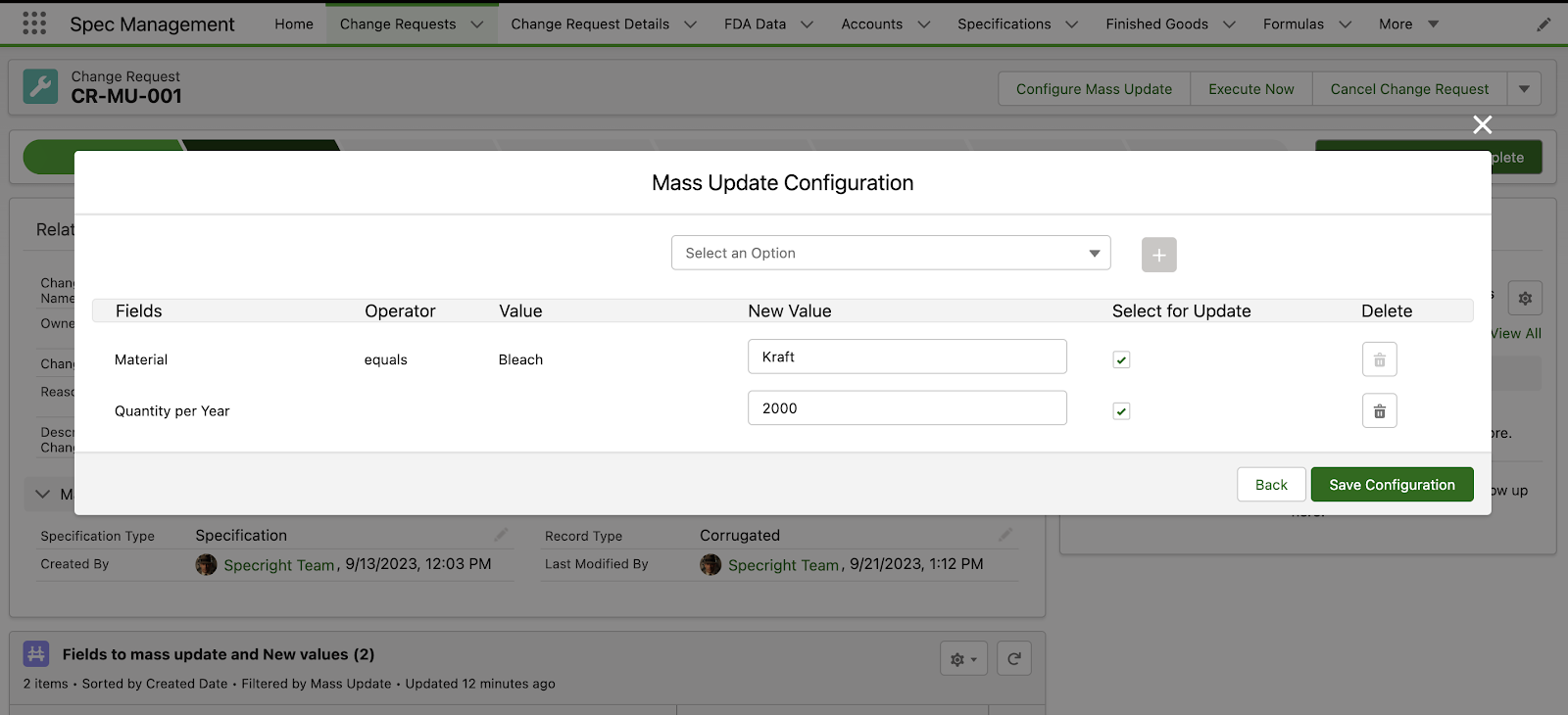
Fig 3: Mass Update Configuration
Plan for Every Part (PFEP) & View Components – Phase 1
As supply chains and product innovation become increasingly complex, we are continuing to provide resources to efficiently manage them. So, we have released a new standard capability to track and manage instructions within Specright. We have also released the first view that will allow customers to build out views for operators to interact with while conducting pack outs.
This feature allows customers to manage their pack-out instructions in the system. This enables Specright to be used downstream by users that we previously might not have had in the system.
Instructions are treated as specifications in the system and can be linked to many different Finished Goods in the system to build out instructions for operators to follow.
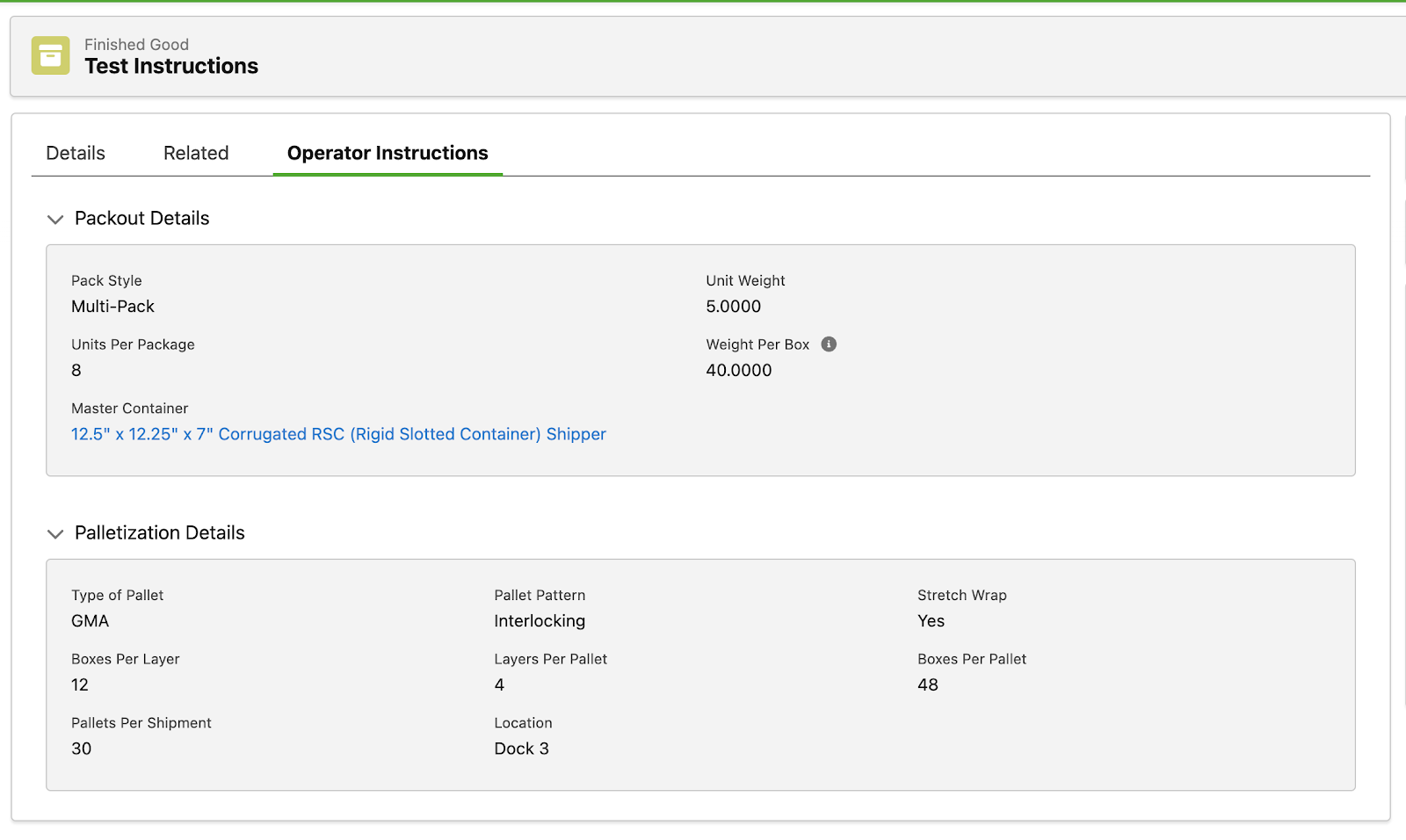
Fig 4: PFEP Field Section
FDA Supplier Recalls/Inspection Data Integration
With more regulations and compliance checks being implemented, it is crucial for our customers to be able to easily pull, analyze, and share their data. One way to do this is with our new integration of FDA Compliance Data into Specright. This provides users with access to critical compliance information related to their facilities and suppliers. This feature aims to enhance regulatory transparency and empower customers to make informed decisions regarding compliance and risk management. This initial integration encompasses the following data categories: Inspection Citations, Inspection Classifications, Import Refusals, and Recalls.
Key benefits of FDA Supplier Recalls/Data Integration includes:
- Critical Compliance Information at Your Fingertips: The FDA Integration feature provides users with direct access to essential compliance data. This includes Inspection Citations, Inspection Classifications, Import Refusals, and Recalls. Say goodbye to time-consuming searches and incomplete information – everything you need is now conveniently available within Specright.
- Supplier Performance Evaluation: Evaluate your suppliers’ performance by reviewing their compliance history and trends. Use this information to make informed decisions about supplier partnerships.
- Customized Alerts: Stay up-to-date effortlessly with customized alerts and notifications related to your facilities and suppliers’ compliance status. Set thresholds and preferences to receive timely information.
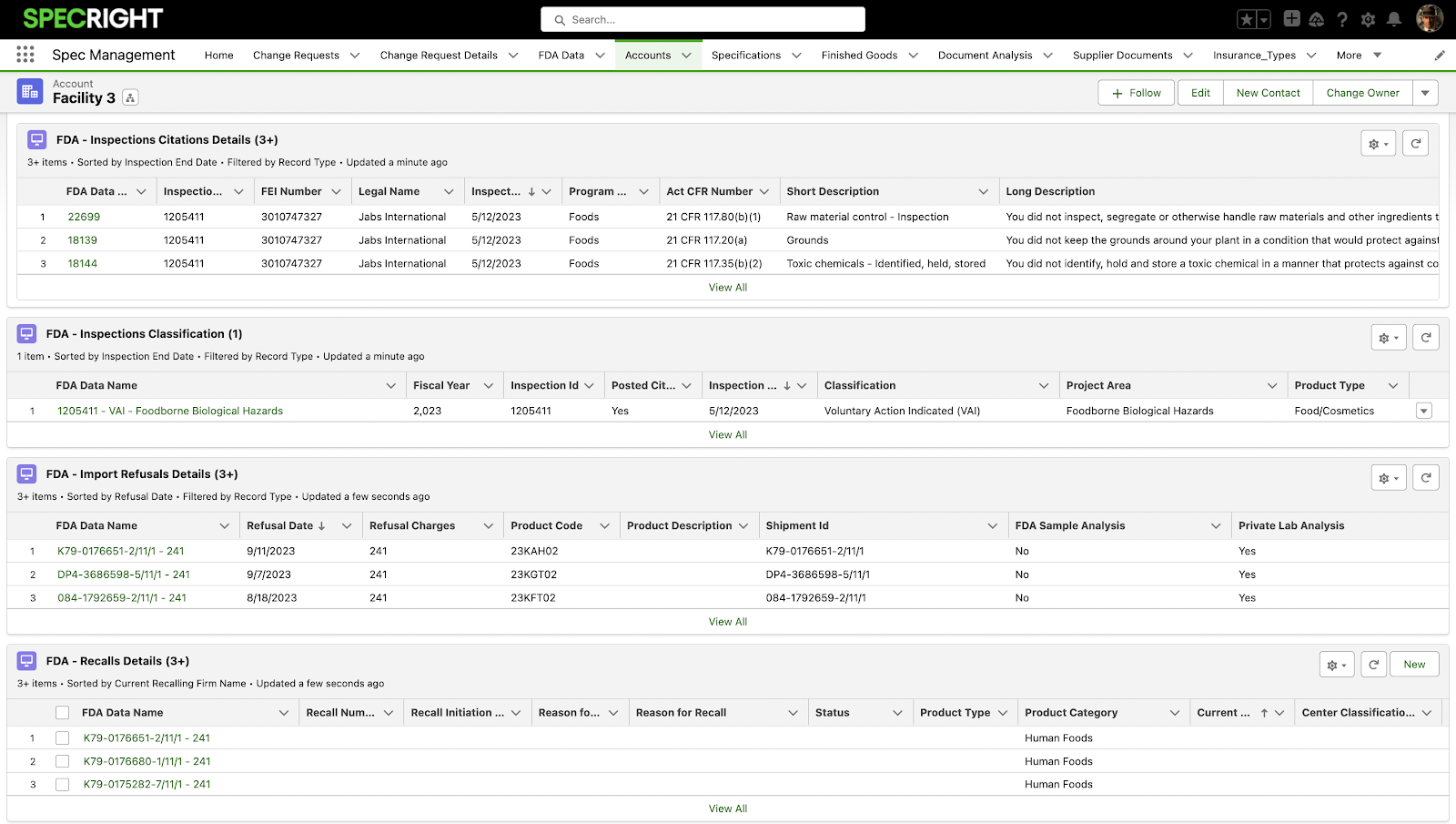
Fig 5: FDA Integration
COMPASS Product LCA
We are expanding upon our last product release by continuing to improve on our partnership with Trayak and its COMPASS solution for LCAs. Specright’s COMPASS integration enables a direct link between Specright and the user’s EcoImpact-COMPASS solution, allowing users to quickly conduct Life Cycle Assessments (LCA’s) on their finished product. The latest version of the integration enables users to quickly run LCAs on their Product BOMs, in addition to the feature’s original Packaging BOM capability.
Users will be able to quickly run Product LCAs, cementing Specright’s role in a company’s sustainability strategy by allowing the export of complete product BOMs (including packaging, ingredients, and other product components) for calculating Product Carbon Footprints and other environmental KPIs on a product level.
This feature is ideal for customers that are interested in streamlining the way their product and/or sustainability teams calculate the environmental impact of their products. Users will save time by not needing to manually enter BOM and material information into their LCA tool.
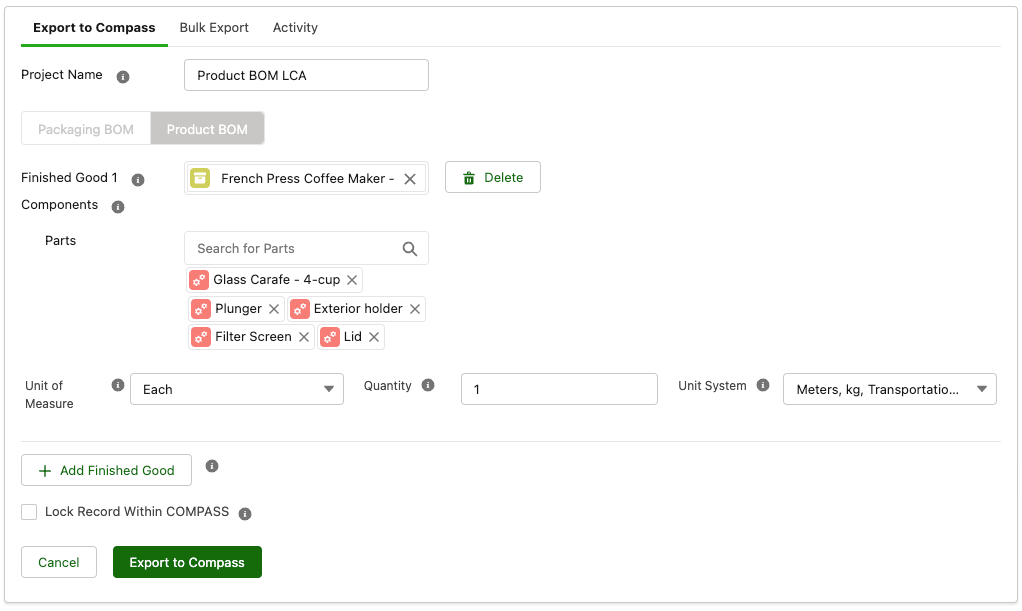
Fig 6: COMPASS Product LCA Export Screen
Improvements to our Specification Data Management Platform
Spec Compare – Related List Comparisons
With this enhancement, the Spec Compare feature now offers the flexibility to compare both parent and child records, providing a comprehensive view of your specification data relationships. For instance, when using Spec Compare on Formulas, you can now effortlessly compare not only the formulas themselves but also their associated ingredients. This feature is designed to streamline your data analysis and decision-making process, ensuring a deeper understanding of your specification data..
This enhancement enables a more comprehensive data analysis, allowing users to understand how parent records are connected to and affect child records, promoting informed decision-making and ensuring that changes align with business goals.
It also streamlines workflow, saving time and effort, and aids in maintaining data accuracy and consistency by quickly identifying discrepancies between related records.
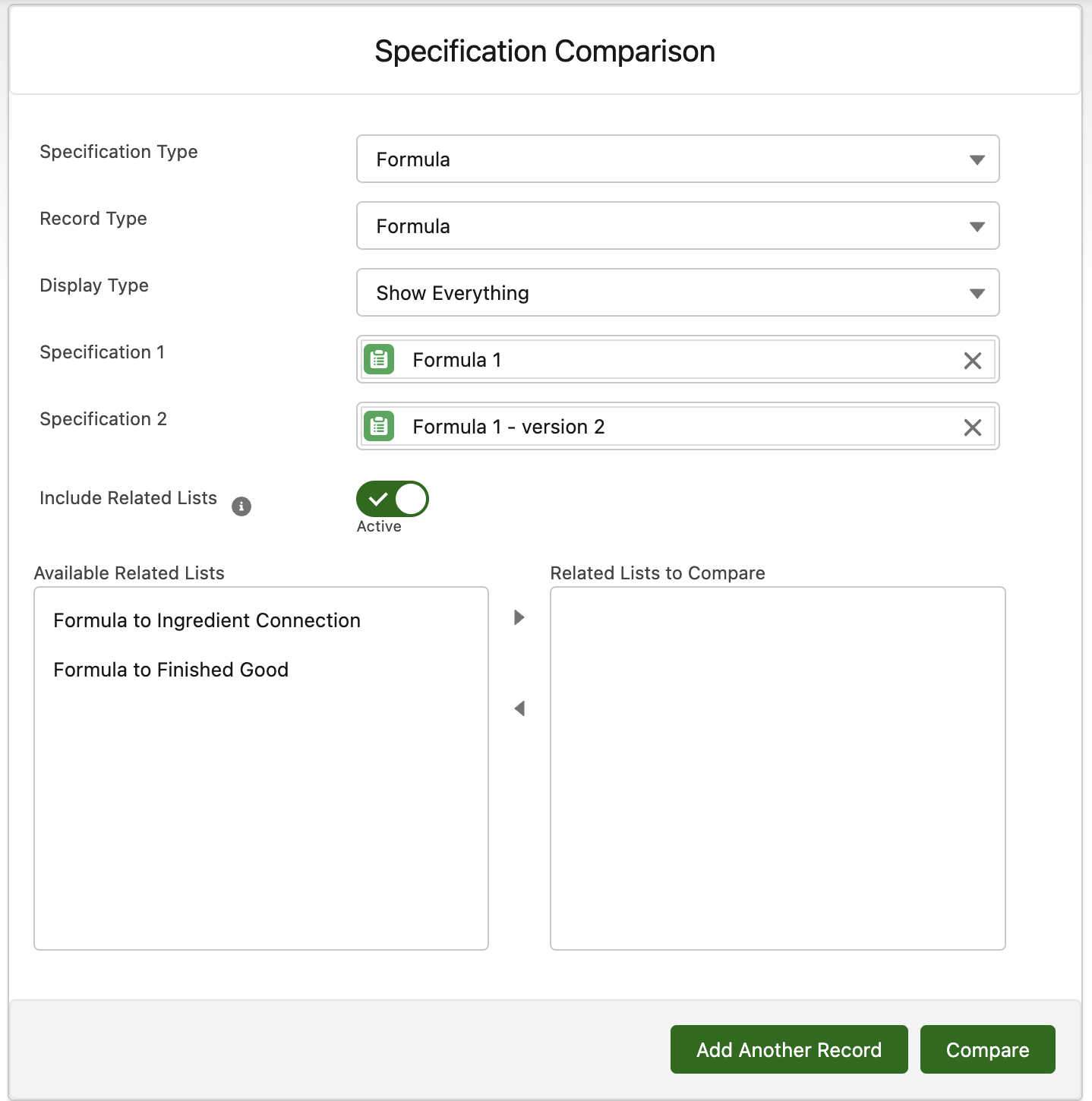
Fig 7: Spec Compare Selection
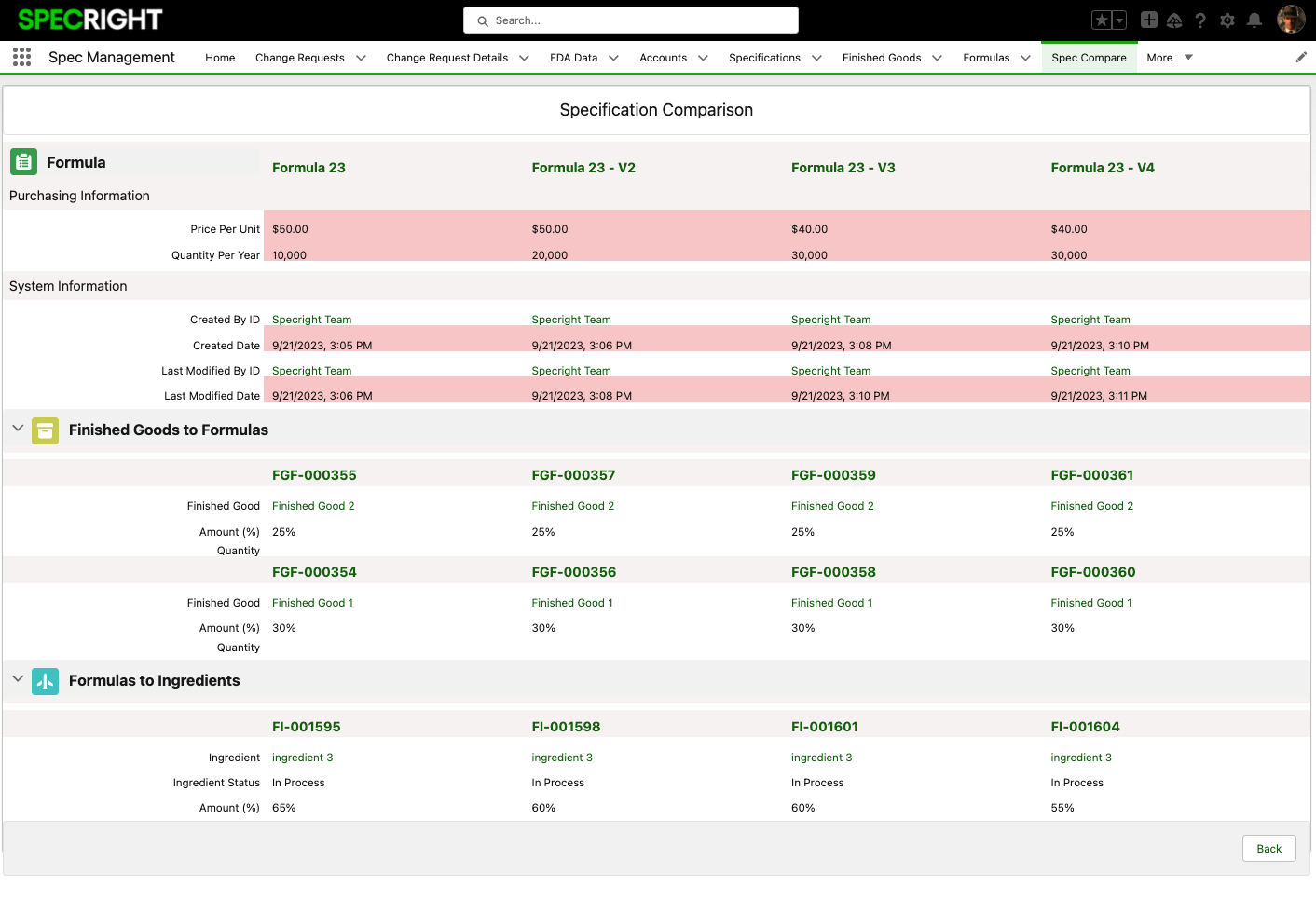
Fig 8: Spec Compare – Final Comparison showing Related Connections
UoM Conversion Enhancements
The Unit of Measure (UoM) feature automatically converts values between paired fields, so that users can add information to specs in the units they use (like inches), while still supporting global businesses by allowing other users to read specs in their preferred units (like millimeters).
In the 23.0 release, we have added two enhancements for admins:
1) Conversion factor override, where admins can change or add more decimal places to the conversion factor used between two units
2) Custom Unit Support, where admins can define conversion factors for field pairings that include at least one field in a unit that is not available in the standard Specright unit list.
In earlier versions of this feature, we defined conversion factors to four decimal places, meaning users sometimes did not get the converted value they expected. With the conversion factor override enhancement, we are enabling admins to adjust conversion factors to be the number of decimal places desired by the customer (up to nine decimal places).
With the addition of Custom units of measure, customers are no longer limited to the units of measure that Specright has defined conversions for. Admins can now use the UoMConversion feature to convert any unit pairings that have a defined conversion factor.
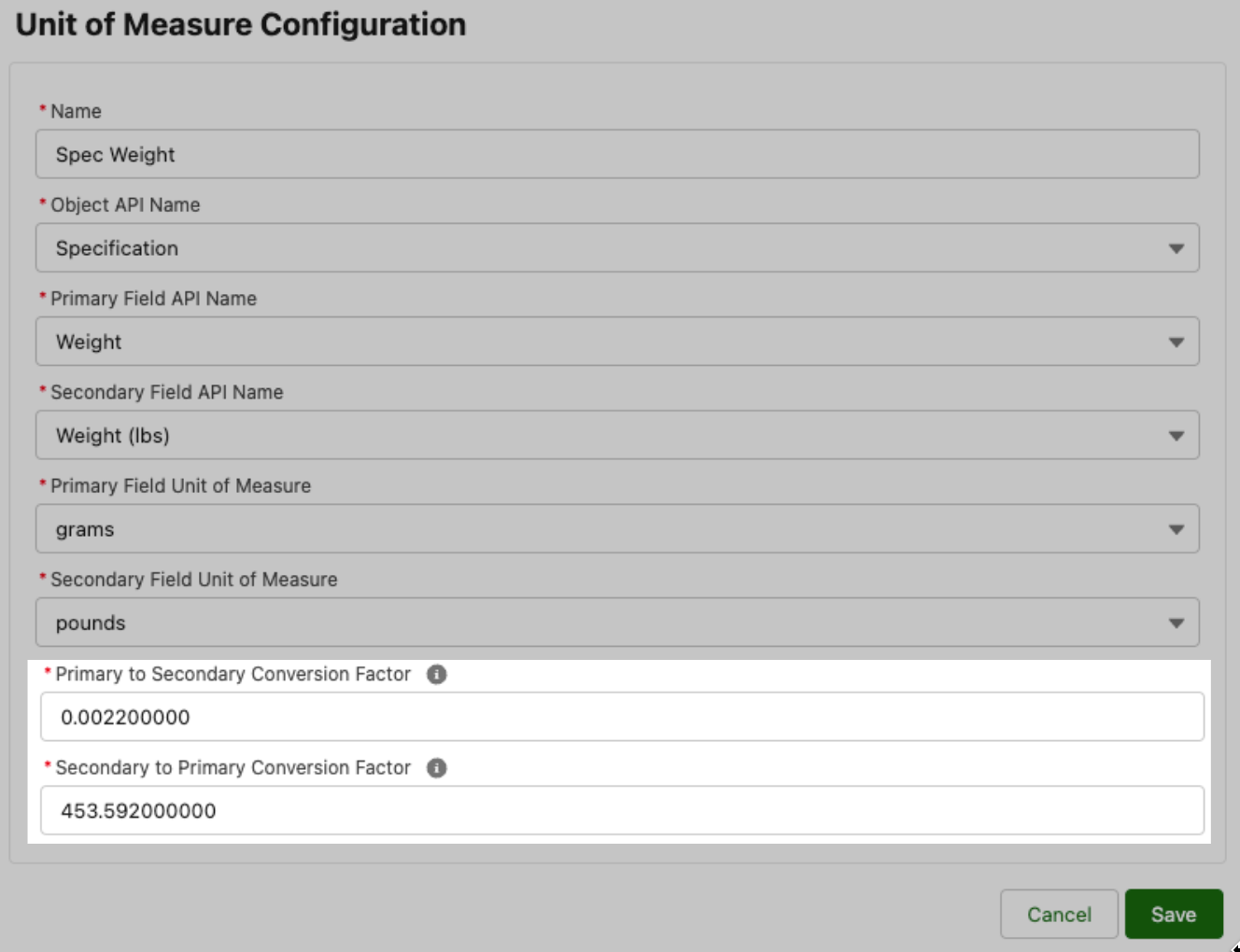
Fig 9: Unit of Measure Configuration
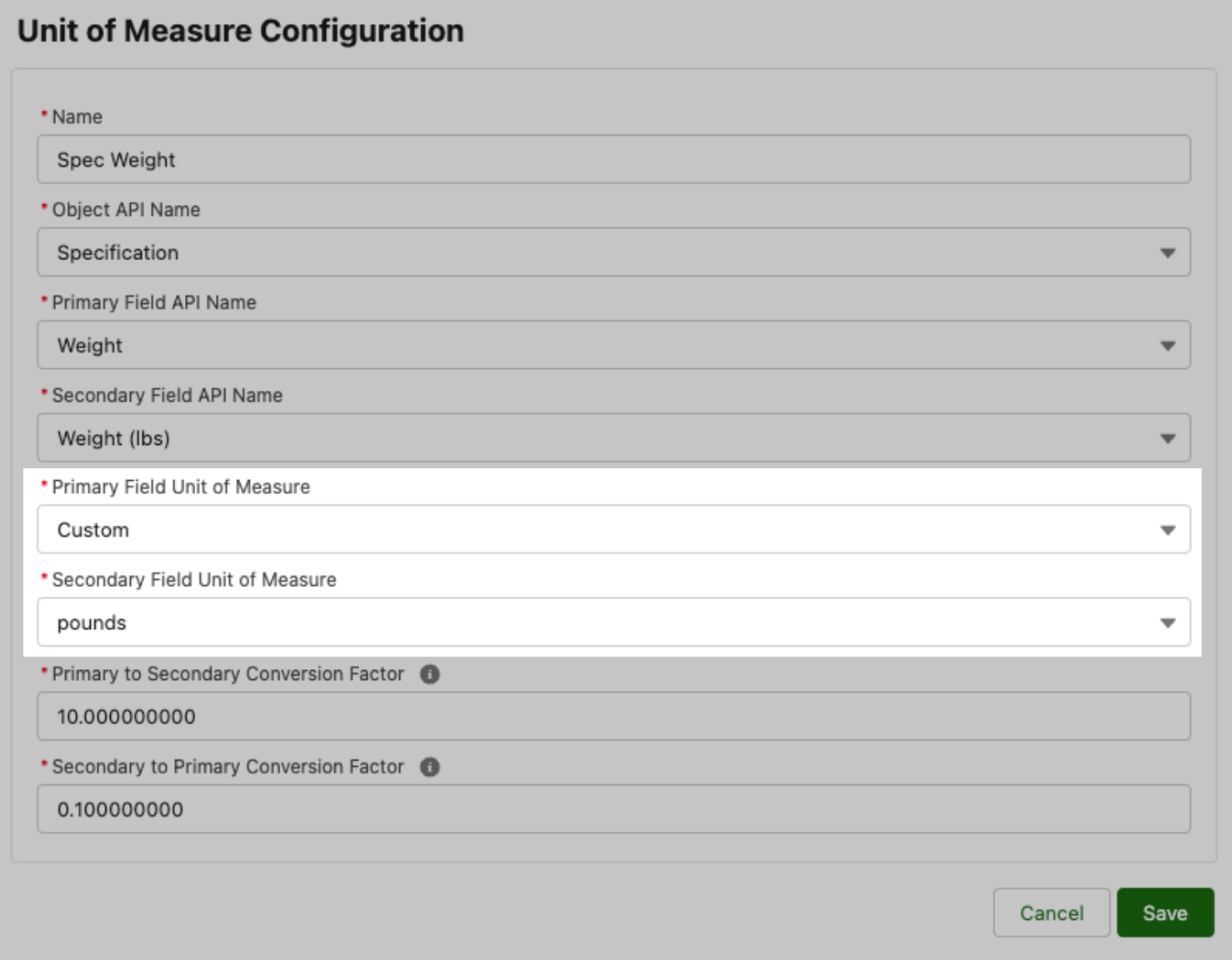
Fig 10: Unit of Measure Custom UoM
Dynamic Clone & Custom Supersede Enhancements
We have updated the Clone and Supersede functionalities to allow for fields in the modal and different related lists. Admins can configure different fields and related lists for various record types.
Key Benefits of Dynamic Clone & Custom Supersede Enhancements:
- This enhancement will allow for admins to control which fields and related lists display when a user is cloning or superseding a record.
- Different related lists by record type allow for the modal to be as accurate as possible for when a user is conducting a clone or supersede. Previously users were provided with all options in the settings for that object, resulting in confusion or errors when cloning and superseding.
- Additionally, allowing fields into the popup when cloning/superseding enables customers to use our managed auto-numbering functionality where it concatenates data from the record itself to generate a unique ID.
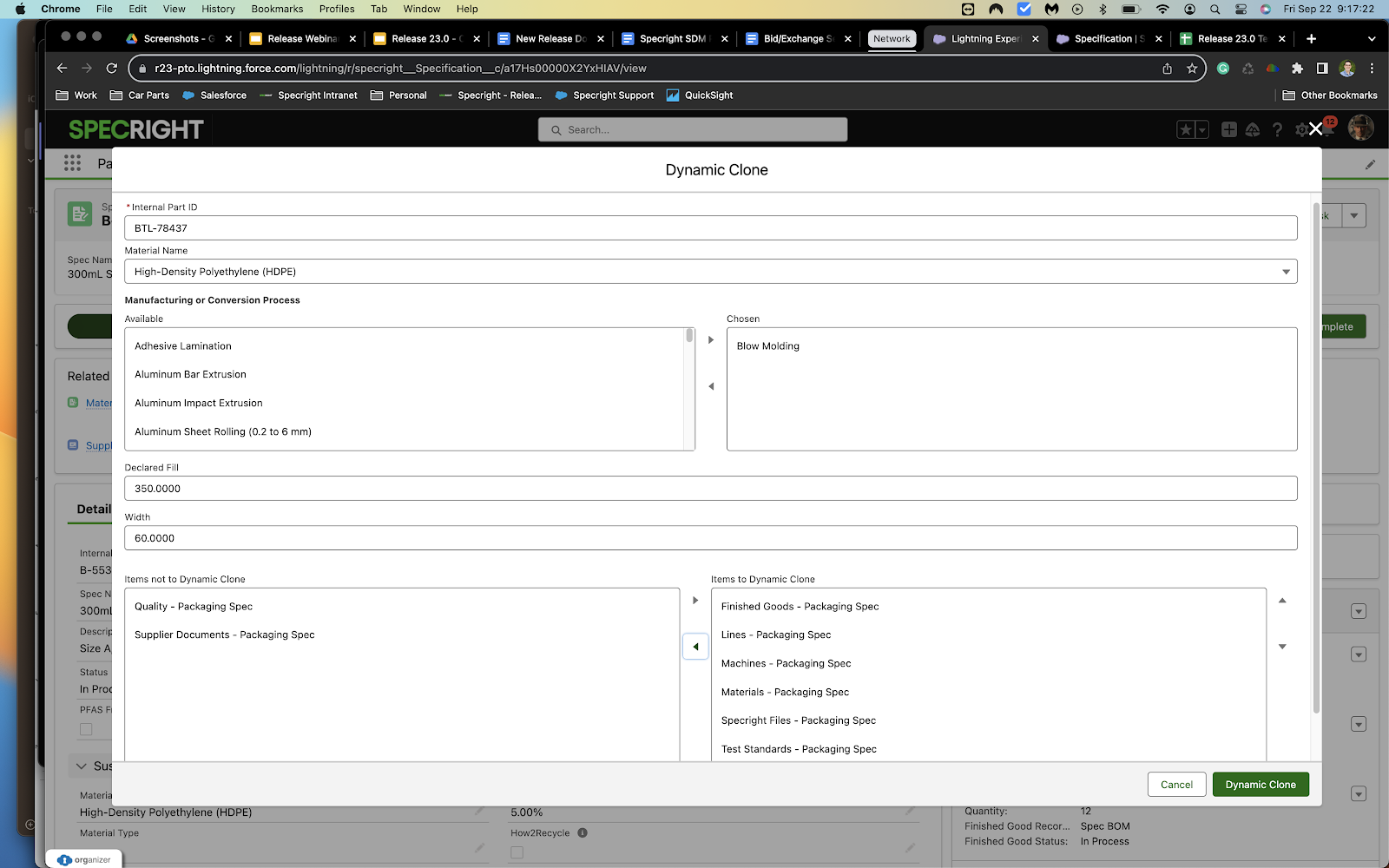
Fig 11: Clone & Supersede Enhancements
IDP Email Submission Capability
Specright’s IDP Email Submission capabilities facilitate communication with suppliers and vendors. This enhancement introduces a streamlined capability within the IDP feature, enabling users to conveniently submit COA or COI documents via email. With this new capability, users can easily request suppliers and vendors to directly send their COA or COI documents to designated IDP email addresses. The system will automatically extract and prepare all the relevant information from the email attachments, facilitating a seamless review process for assignment administrators.
This feature simplifies the submission of COA or COI documents by allowing users to send them via email, reducing the complexity of manual processes. It also streamlines communication with suppliers and vendors, ensuring that required documents are received promptly.
Moreover, the automation of information extraction from email attachments saves users valuable time, making the process more efficient, especially in time-sensitive situations. The feature further enhances the review process by automatically sending an email to the assigned reviewer.
Overall, this feature greatly improves the user experience, increases accuracy, and provides an efficient way to manage critical documents, with the added benefit of an audit trail for compliance and record-keeping purposes.
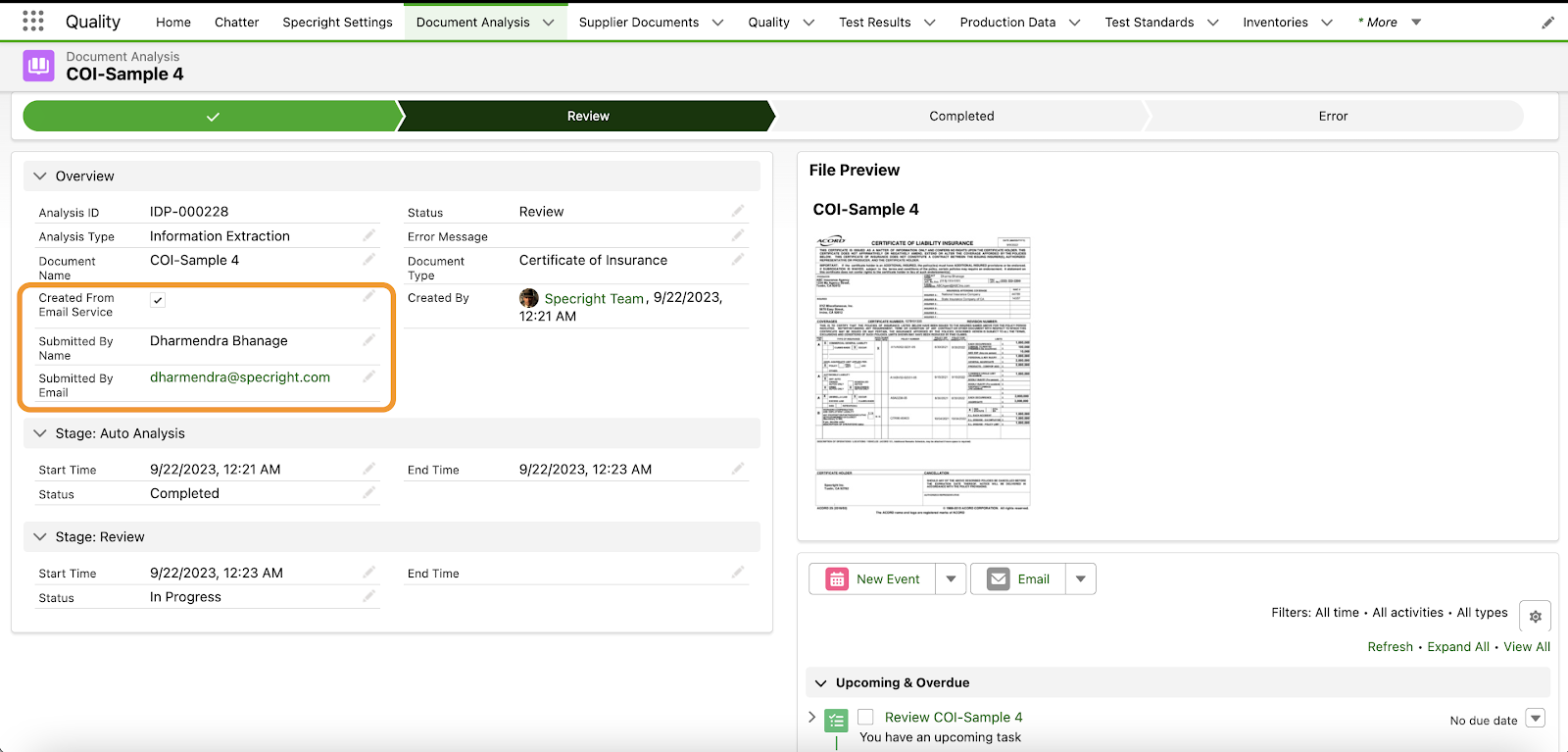
Fig 12: IDP Document Analysis – New Fields for Email Submission Option
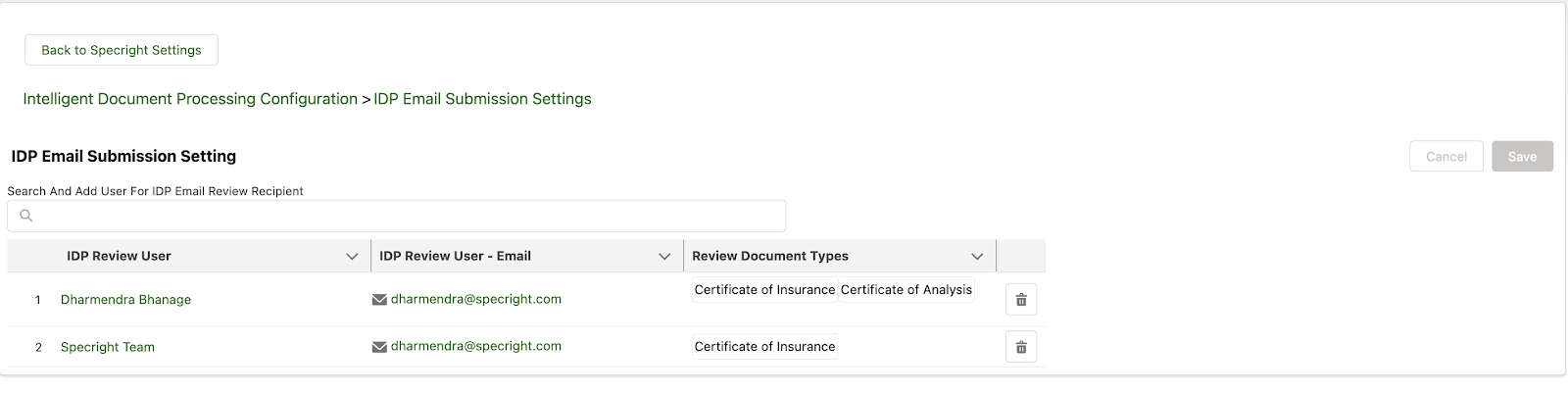
Fig 13: IDP Email Submission Settings – Reviewer for Emailed Docs
Updates to Sustainability YoY Benchmarking Reports
In this release, we’re excited to introduce an enhancement to our sustainability reporting feature. Building on our previous release, which introduced the capability to capture specific sustainability fields year over year through a user-friendly interface, we’re taking it a step further in this release.
Users now have the flexibility to expand beyond the standard set of sustainability fields. They have the freedom to incorporate additional sustainability fields that are directly relevant to their unique company and industry.
Key Benefits of Updating the Sustainability YoY Bencmarking Reports:
- Tailored Sustainability Tracking: Users can now align their sustainability tracking precisely to their company’s specific goals and industry requirements. This customization ensures that the sustainability data collected is highly relevant to their unique circumstances.
- Flexibility for Changing Needs: As a company’s sustainability goals evolve over time, this feature provides the flexibility to adapt and add new fields as needed. It accommodates changes in sustainability priorities without requiring a major overhaul of reporting systems.
- Industry Compliance: Companies operating in highly regulated industries can use this feature to ensure that they are capturing and reporting on all the sustainability metrics required for compliance with industry regulations and standards.
Specright Specification Data Management Enhancements
- Visual Hierarchy Connections: An issue with Visual Hierarchy meant that wrong connections were being displayed upon expansion of a branch. This has been fixed.
- Custom New Record Page: We have addressed a couple of issues with custom new record pages – First one supports required fields on these custom pages. The second one is fixing the behavior of decimal fields on these pages to mimic standard page behavior.
- History Tracking User Locale: The History Tracking date fields was not displaying the correct user locale earlier. This has been addressed.
- Salesforce Files – View File Link: The View File link was not generating correctly for customers that used Salesforce Files. This has now been resolved.
- TOPS and COMPASS Integration Issues: Several minor issues on these integrations have now been resolved.
As mentioned earlier these are only a few of the new capabilities and enhancements in Release 23.0. If you’re interested in learning more, watch our on-demand webinar here. You can always reach out to our team to learn more. Your support motivates our team to keep innovating and we hope you enjoy the updates.
Until the next release!
Explore More Blogs
Get Started
With Specright’s Solution Suite, you can digitize, centralize, and link your specification data to drive efficiencies, intelligence, traceability, and collaboration within your organization and across your supply chain network.



