Quality Management
Streamline quality processes to resolve issues faster
Manage every quality process in one place, from incident intake to reporting, with built-in automation and dashboards.
Why Specright for Quality Management
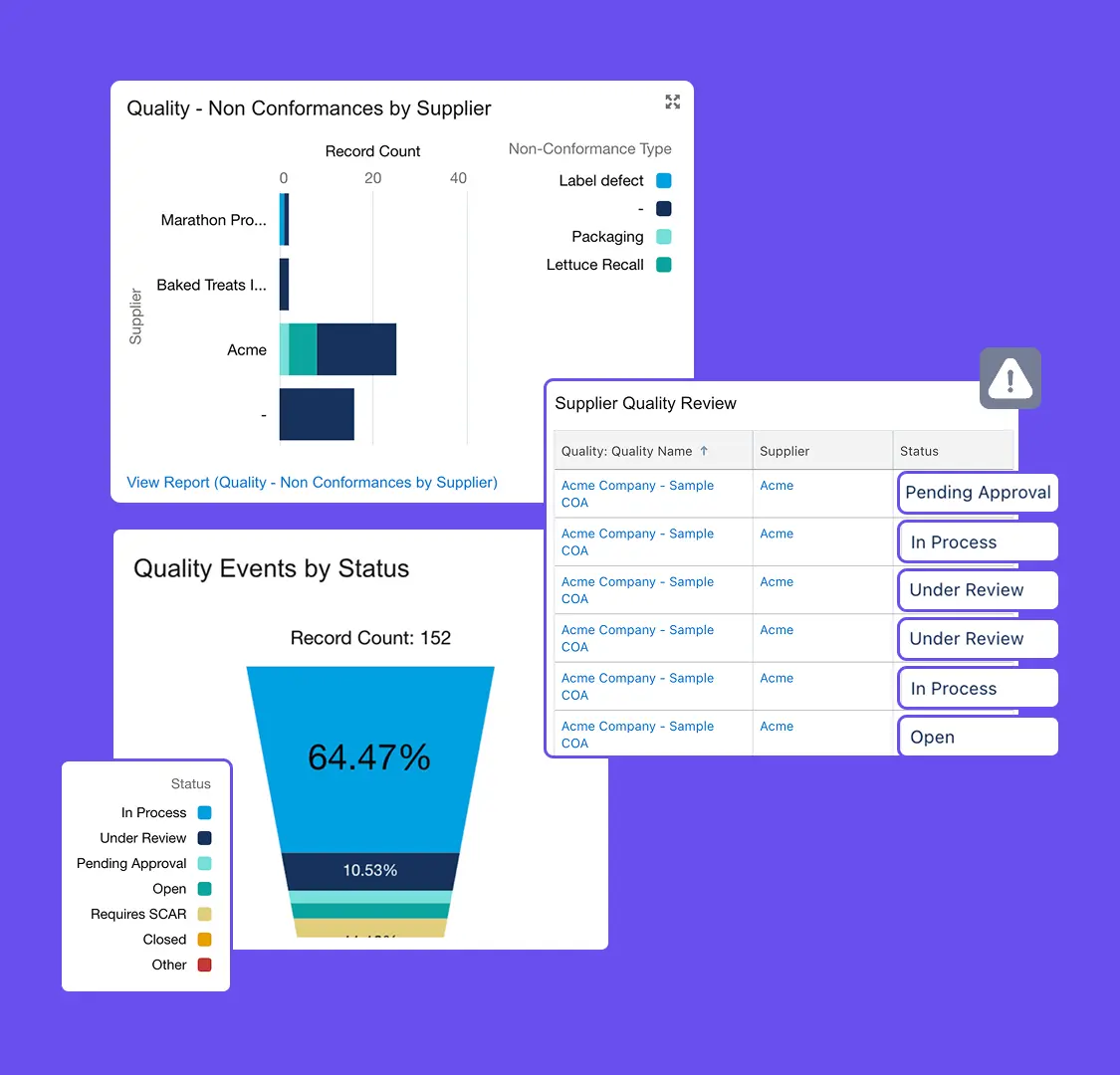
Centralize Quality Data
Manage incidents, audits, and corrective actions in one system for full traceability.
Resolve Issues Faster
Automate quality workflows and approvals to speed incident resolution.
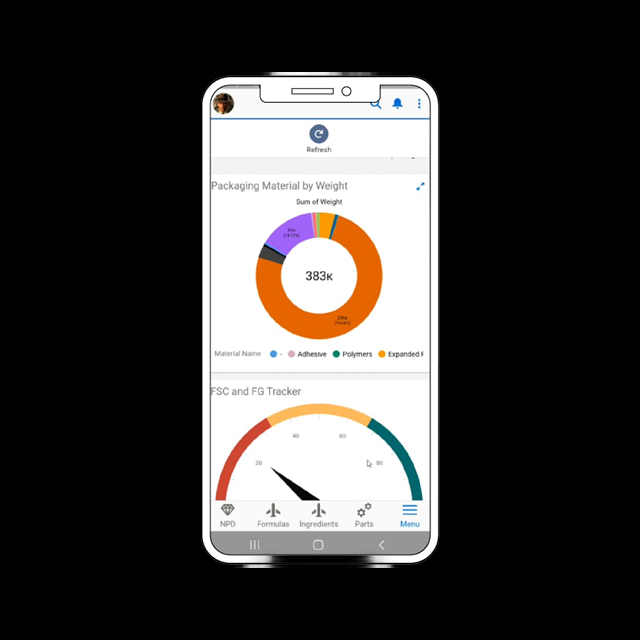
Monitor Quality Trends
Track issues and supplier performance with real-time dashboards and reports.
Trusted by Fortune 500, private label retail & challenger brands











Key Features
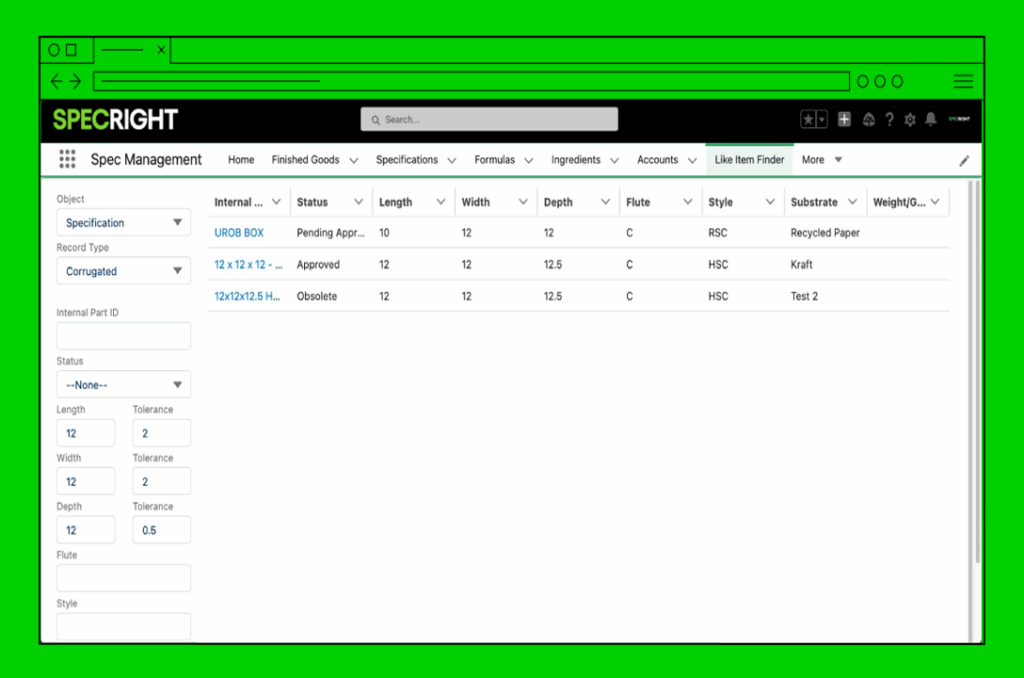
SCAR, CAR, Non-Conformance
Quickly create and link actions to the right spec or product to identify, correct, and prevent issues.
COA Management
Manage Certificates of Analysis in one place to easily verify incoming materials meet standards.
Test Results & Attributes
Set requirements, capture results, and trace at the lot level to ensure products meet specs.
Impact Metrics
30%
Reduced Risk of Recalls
15%
Faster Return of Market for Recalled Products
40%
Fewer Rejected Shipments
30%
Faster Preparation of Regulatory Audits
What Product Experts Say

“I no longer have to search
for information and validate the source—I grab
the information I need from Specright and
move on with my project.”

Laura Berlanga
Product Innovation & Research Manager, Ocean Mist Farms

“Being able to review specs
with sales data is changing
the way we do business.”

Molly Fiedler
Chief Innovation Officer, Kira Labs

“Having more people access data
allows us to put world class operating procedures in place.”

Kris Corbin
Chief Supply Chain Officer, Bright Future Foods

How to Use Data to Prevent Quality Issues in Your Supply Chain eBook
Recalls, incorrect orders, and product waste all stem from one fundamental problem: the way businesses are approaching quality management.
Discover the Impact
If you get the spec right,
the rest follows
With digitized, shareable specs, you can ensure your teams and suppliers are on the same page– at all times.