Formulation Software for Any Stage of the Product Lifecycle
Product specification management is the discipline of managing all of the data needed to make a product and its related components, such as packaging, raw materials, formulations, and ingredients.
Why Specright for Product Formulation?
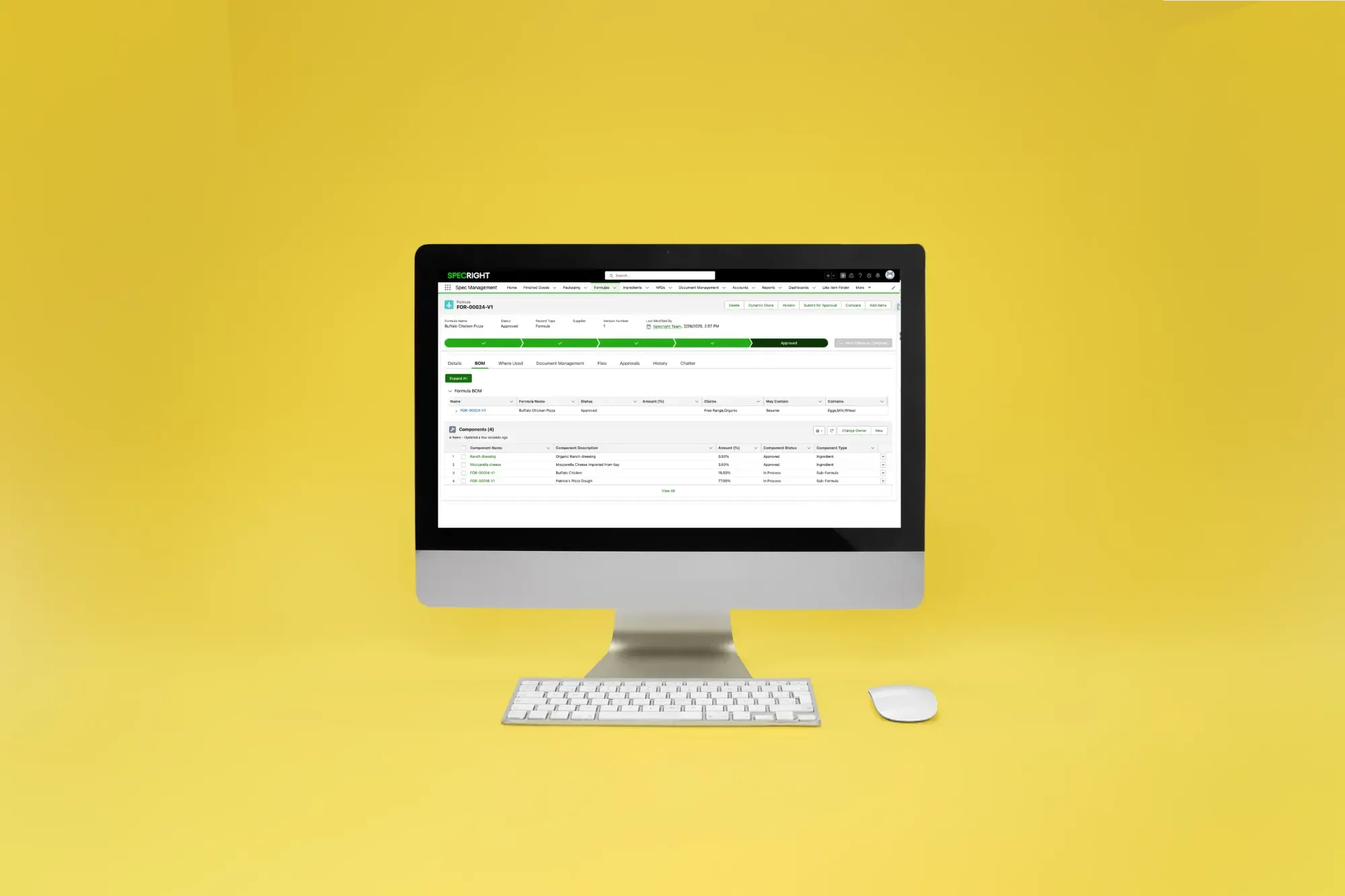
Product formulation software helps businesses develop products quickly and cost-effectively by providing all relevant data in one centralized system. Specright’s formulation architecture ensures best practice management for ingredient, formula, and finished good data.
In Specright, the ability to connect a single ingredient to multiple formulas and finished goods means any change to an ingredient is instantly reflected in all associated formulas and finished goods, reducing errors, enhancing compliance, and accelerating time-to-market.
- Consolidate complex formulation data into a single, intelligent platform
- Ensure consistency and traceability with comprehensive ingredient data management from formulation to shelf
- Leverage insights to optimize ingredient selection and performance
- Enable real-time collaboration across global operations
Manage Simple & Complex Formulations with Specright
With Specright, you can link ingredients together to quickly build formulas. For more complex formulations, you can nest formulas within other formulas to accelerate product development. Throughout the product development process, you can create new formulas and ingredients or utilize existing formulas and ingredients already stored in your Specright instance.
Have an existing formula you just need to tweak? With our cloning capabilities, you can easily copy and create new variations. And we know things change and history is important – so you can easily supersede formulas and track previous versions and field changes – all on Specright.
- Clone formulas to easily create a new variation
- Versioning formulas and easily track previous versions
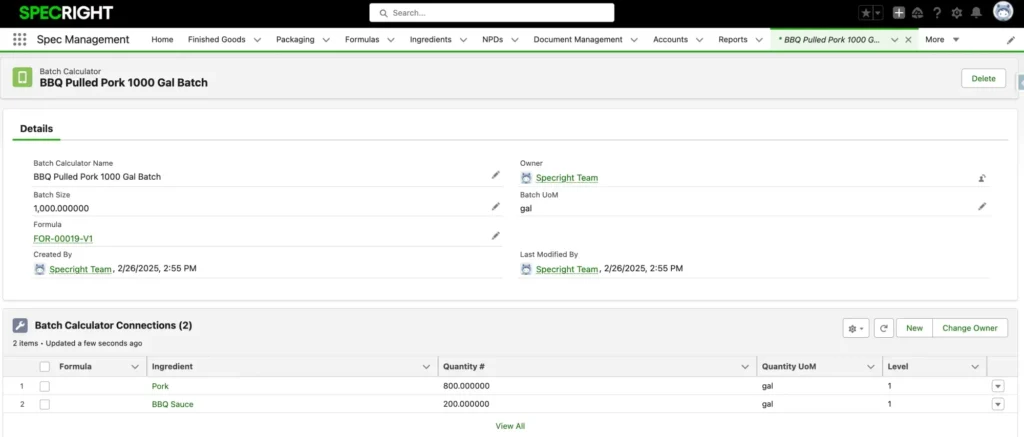
Streamline Batch Scaling and Production
Eliminate manual scaling complexity by enabling instant batch size adjustments and multiple batch scenarios within a single record, empowering more agile and accurate production processes. Specright’s Batch Calculator dynamically scales formulas while maintaining perfect compositional accuracy through automated quantity calculations and unit conversions.
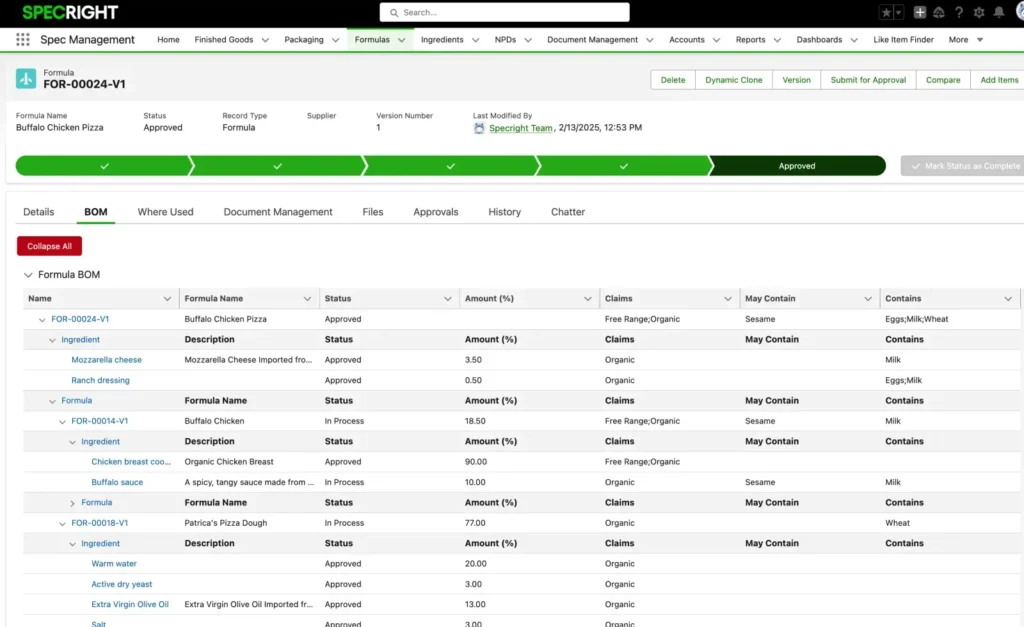
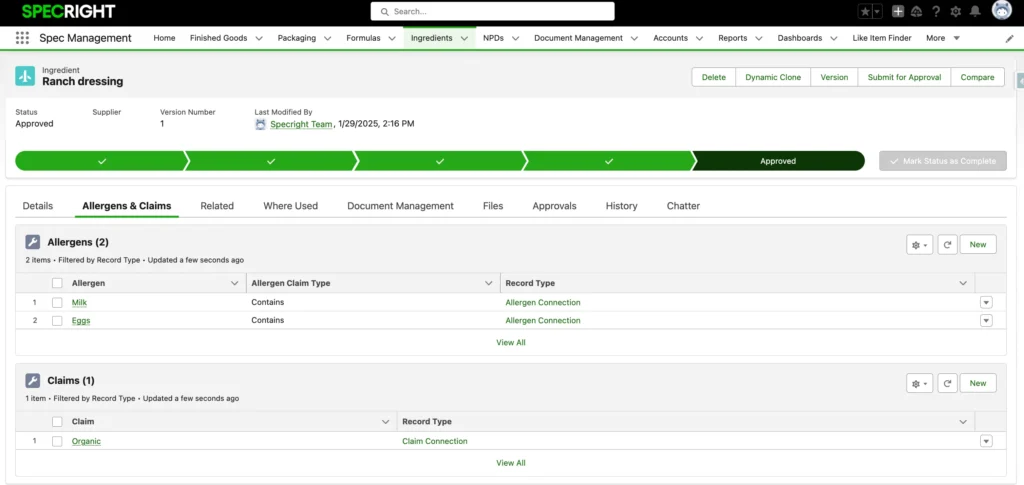
Track Allergens & Claim Modifications Seamlessly
Automate the tracking and roll-up of allergen declarations across your entire product portfolio. Eliminate manual allergen tracking complexity by implementing smart inheritance logic that automatically propagates allergen claims through ingredient-formula-product relationships, enabling proactive risk management and enhanced product safety documentation.
Define sophisticated claim inheritance rules and implement direct claim overrides, enabling more accurate claims tracking and flexibility across all product levels.
Claims are managed alongside allergens and their connections, using dedicated record types. Users can define logic to control how claims roll up through hierarchical relationships, such as from ingredients to formulas or products.
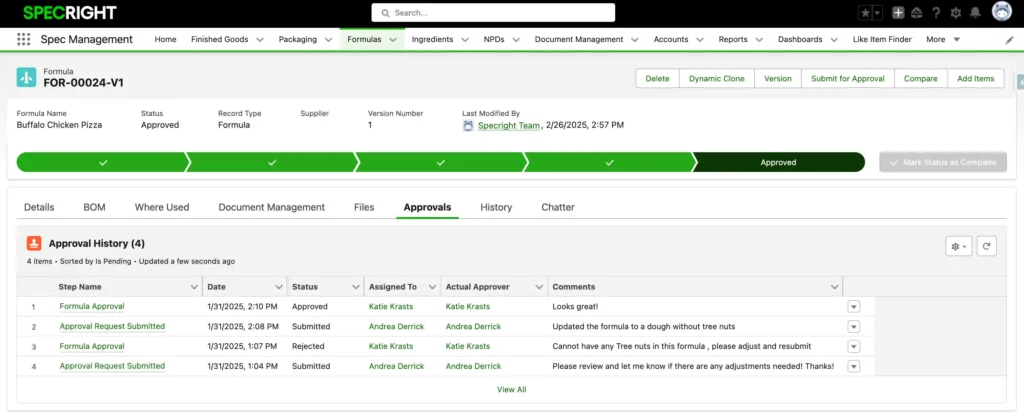
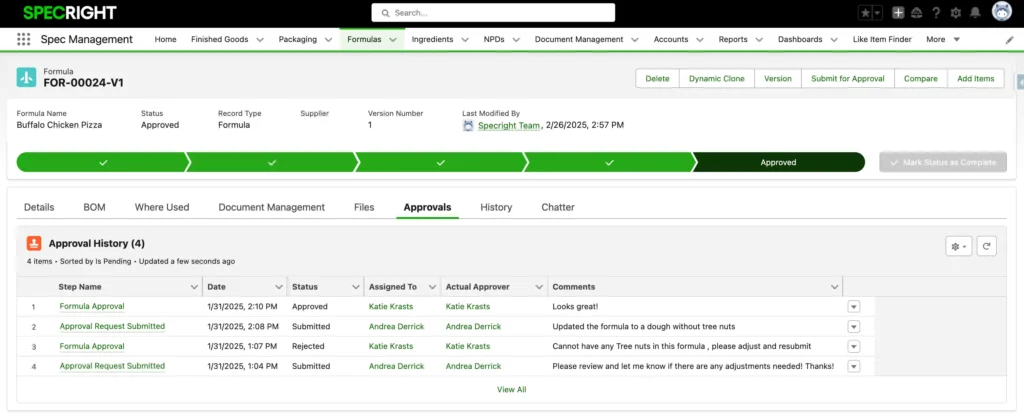
Track Every Step of the Formulation Approval Process
Specright streamlines formula management with automated routing, comparison tools, and secure data controls.
- Route formulations through stage gates with real-time stakeholder approvals (including partners in your supply chain)
- Compare formulas side-by-side to identify changes with Spec Compare
- Protect sensitive data with advanced user permissions
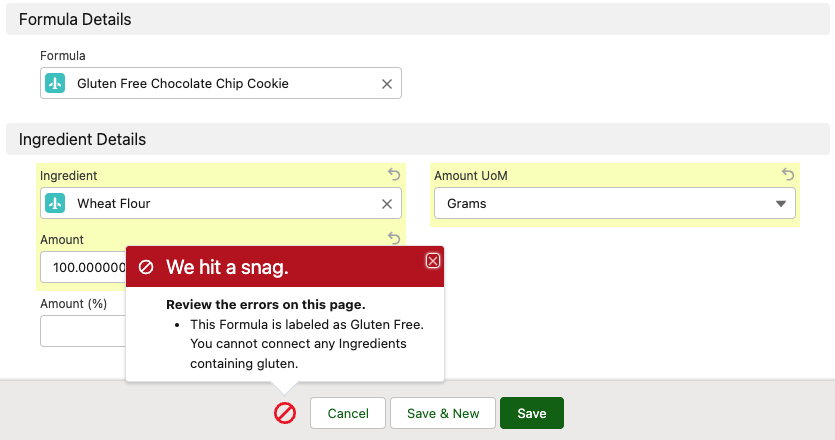
Determine Compatibility by Linking Formulas to Products & Packaging
Some ingredients are incompatible with products or packaging due to allergens, material conflicts, or regulatory restrictions in certain countries. When you link formulas to products in Specright, you’re alerted of potential conflicts which can help avoid potential recalls, taxes, or product failures.
Link Formulas to Finished Goods and Packaging Specifications to determine compatibility.
Other Formulation Management Systems
Clunky Integrations
- High implementation costs and lengthy setup times.
- Risk of data inconsistency due to lack of standardization across systems.
- Limited flexibility to adapt to new business needs or supplier changes.
- Inefficient communication and collaboration between systems, leading to delays and errors.
Do-It-Yourself
- Time-intensive and prone to errors due to manual processes and disconnected spreadsheets.
- Lacks scalability and standardization, creating data silos.
- Limited reporting and analytics capabilities for compliance and optimization.
- Requires significant internal resources for maintenance and updates.
Other Formulation Software
- Outdated UI and poor user experience creates inefficiencies.
- Limited capabilities for full specification management; primarily focused on ingredients.
- Collaboration restricted to ingredient suppliers only.
- Offers CAR, SCAR, and COA reporting but lacks automation and advanced analytics.
Specright: The Smarter, More Connected Approach to Formulations
- Centralized System: Centralized platform for managing all types of specifications, including formulas, ingredients, packaging, and finished goods.
- Gartner Category Leader: Named the category leader by Gartner Research.
- Supplier Collaboration: Collaboration with any 3rd party supplier or manufacturer, not limited to ingredients.
- Supplier Portal: Suppliers can enter ingredient specifications directly via the supplier portal, streamlining collaboration.
- Robust Reporting Tools: CAR, SCAR and COA reporting, plus Specright’s Intelligent Document Processing automates data ingestion and flags non-compliance via alerts & reports.
- BOM Management: Intelligent BOM management seamlessly links specifications across the supply chain.
Get Started
With Specright’s Solution Suite, you can digitize, centralize, and link your specification data to drive efficiencies, intelligence, traceability, and collaboration within your organization and across your supply chain network.